Chromidina chattoni
Diagnosis
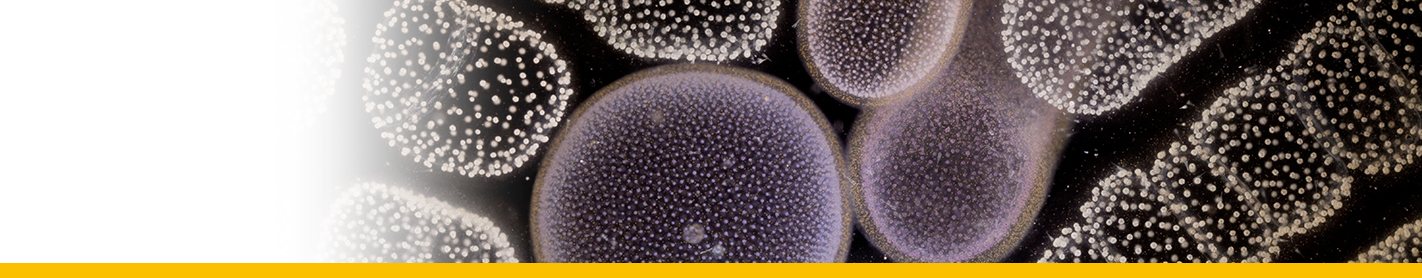
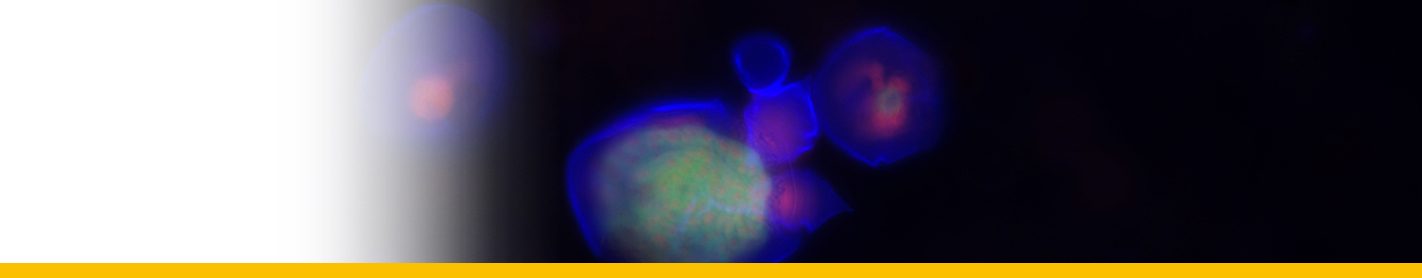
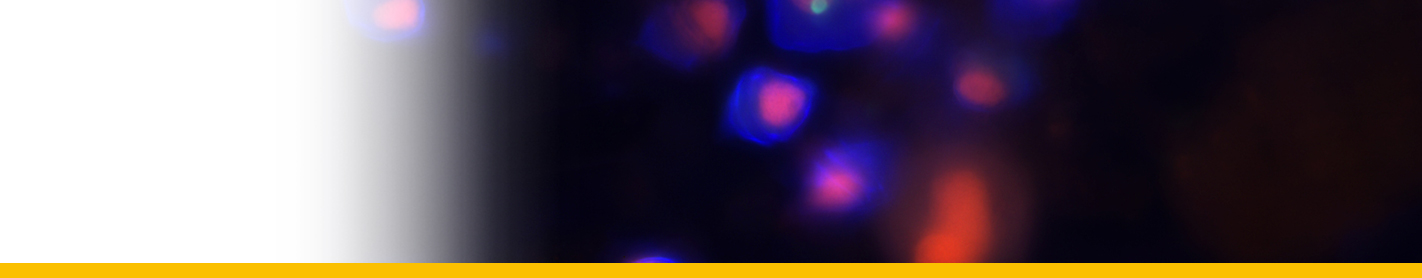
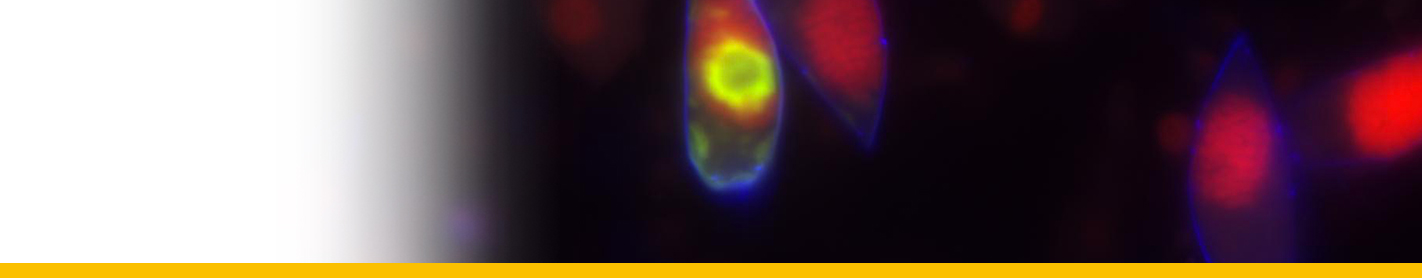
Diagnosis_Species: Tropho-tomont body: Elongated and thin vermiform shape. Body width constant along body length (mean = 19.5 ± 3.7 µm, N = 60). Cytoplasm filled by darkly-stained islands of chromatin. Small trophotomonts weakly stained by haematoxylin, no clear network or spots of chromatin observed, suggesting degenerative forms rather than apotomite forms. Haematoxylin stain of tropho-tomonts revealing typical reticulated macronucleus of Chromidina, spreading throughout the entire cell body. Anterior end: globular with regular width (mean = 40.8 ± 4.7 µm, N = 52). Typical bulb-like anterior end, but heterogeneous in length of 80–1,890 µm (mean = 657 ± 486 µm, N = 33). No distinguishable apical papillum observed. One to two large and darkly-stained spots of chromatin often associated with the bulb-like anterior end. Ciliature: Consisting of 13 dextrally-spiralled kineties originating from the apex and continuing uninterrupted on the entire cell body. Posterior end: With typical Chromidina division segments that could generate either apotomites by monotony division or tomites by palintomy division.
Body_trophonts_length: 80-1,890 µm
Body_trophonts_width: 19.5 + 3.7 µm
Kineties_total: 13
Sequences_SSU: LT546660, LT546661, LT546662
Etymology
named after Edouard Chatton
Type species
Chromidina elegans (Foettinger, 1881) Gonder, 1905
Type illustration / Type locality / Type specimen
Type illustration: https://commons.wikimedia.org/wiki/File:Parasite160019-fig2_-_Chromidina_spp._(Oligohymenophorea,_Opalinopsidae),_parasites_of_cephalopods_of_the_Mediterranean_Sea.png
Type locality: Off Tunis, Tunisia, Mediterranean Sea, 364909.1100 N, 1018022.4900 E
Type specimen: Hapantotype catalogued under No. MNHNIR-2016-326 and parahapantotypes catalogued under Nos. MNHN-IR-MNHN-2016-327 to 341, deposited in the Protist Collection of the Muséum National d’Histoire Naturelle, Paris, France
Type material: Haematoxylin-stained smears from the three infected Loligo vulgaris
Type Host: Loligo vulgaris Lamarck, 1798
Zoobank-link: urn:lsid:zoobank.org:act:A533901D-8325-4412-AF74-C63341DB03C7
Ecology
Parasite found in renal sacs of Cephalopods. Chromidina chattoni type host is Loligo vulgaris. Chromidina chattoni has not yet been described in other hosts.
Life cycle
Life Cycle: Chromidina spp. have a polymorphic dixenous life cycle, with two different budding processes which are monotomy and palintomy. The adult stage, the vermiform tropho-tomont, has a maximum body length varying from 400 µm to 2,000 µm. Some rare adult stages can have an accelerated growth process. Their size increases so quickly that their length can measure up to 5,000 µm. Given their unusual extended size, these adult stages are called hypertrophonts. The tropho-tomont is uniformly ciliated and has no cytostome. It is attached through its anterior end to the host kidney tissues with its body bathing in the renal fluids, and feeds by nutriment absorption from host cells and fluids. Division by monotomy produces a single long bud from the posterior end, the apotomite, which is morphologically similar to its parent and develops into a second generation of trophotomonts after detachment and colonisation of the host kidney. Division by palintomy produces smaller buds that form a typical chain of individuals attached to the tropho-tomont, which differentiate into tomites. Budding occurs only from the posterior end. The tomite is a small ciliate form with a unique ciliature and a cytostome. When detached, it is believed that the tomite leaves the renal appendages to be released with passage of urine into the sea. This stage is presumed to encyst, as a phoront, and to infest an intermediate host. However, no intermediate host has been confirmed so far. In Souidenne et al., 2016.